REVIEW ARTICLE |
https://doi.org/10.5005/jp-journals-11002-0025 |
Role of the Endothelium in Neonatal Diseases
1Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, United States of America
2Global Newborn Society, Clarksville, Maryland, United States of America
Corresponding Author: Olachi J Mezu-Ndubuisi, Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, United States of America, Phone: +6082638558, e-mail: olachimezu@pediatrics.wisc.edu
How to cite this article: Mezu-Ndubuisi OJ, Maheshwari A. Role of the Endothelium in Neonatal Diseases. Newborn 2022;1(1):44–57.
Source of support: The study was made possible by the McPherson Eye Research Institute’s Retina Research Foundation Edwin and Dorothy Gamewell Professorship Award (to OJM), and NIH awards 1K08EY032203-01 (to OJM) and HL133022 and HL124078 (to AM).
Conflict of interest: None
ABSTRACT
In both fetal and neonatal physiologic and pathologic processes in most organs, endothelial cells are known to play critical roles. Although the endothelium is one of the most ubiquitous cell type in the body, the tight adherence to the blood vessel wall has made it difficult to study their diverse function and structure. In this article, we have reviewed endothelial cell origins and explored their heterogeneity in terms of structure, function, developmental changes, and their role in inflammatory and infectious diseases. We have also attempted to evaluate the untapped therapeutic potentials of endothelial cells in neonatal disease. This article comprises various peer-reviewed studies, including ours, and an extensive database literature search from EMBASE, PubMed, and Scopus.
Keywords: Angiogenesis, Bronchopulmonary dysplasia, Endothelium, Necrotizing enterocolitis, Neonate, Retinopathy of prematurity.
IMPACT
We reviewed the scope of endothelial cell heterogeneity, along with the endothelial cell structure and function as seen in the fetus and neonate.
Endothelial cells are a diverse subtype of cells and play vital roles in innate immunity, angiogenesis, tissue homeostasis, repair of tissues, tissue inflammation, and cellular apoptosis in numerous inflammatory and infectious diseases.
Evolutionary mechanisms regulating endothelial cell heterogeneity vary in vivo and ex vivo.
Endothelial cells are important therapeutic mediators in the vasculature of numerous neonatal disorders.
INTRODUCTION
Endothelial cells are metabolically active cells bordering the blood vessels inner lining, where they have a crucial function in both physiology and pathology. Due to their critical anatomic location, these cells have always been believed to have unlimited therapeutic potential, but the relative inaccessibility of the endothelium in intact organs has curtailed detailed in vivo studies. Recent advances in diagnostic microtechnology have provided some solutions to this problem, at least in larger blood vessels, and have renewed the scientific interest in these cells. These cells are important regulators of trans-vascular blood-to-tissue barrier to macromolecules and nutrients, trafficking of leukocytes between blood and inflamed tissues, and of tissue respiration via both hemodynamic homeostasis and neoangiogenesis. With the dispersive, arboreal vascular arrangements, endothelial cells are distributed throughout our body.
Abnormalities in the function of endothelial cells are depicted in several neonatal conditions, such as intraventricular hemorrhage (IVH), retinopathy of prematurity (ROP), hypoxic-ischemic encephalopathy (HIE), bronchopulmonary dysplasia (BPD), acute kidney injury (AKI), and necrotizing enterocolitis (NEC). Endothelial markers may be helpful in the diagnosis, monitoring, prognosis, and clinical management of many neonatal conditions. Therapeutic targeting of microvascular structure and function may also be useful in neonatal conditions. The current article merges peer-reviewed evidence arising from our research as well as extensive literature review from notable databases, such as Scopus, EMBASE, and PubMed.
ORIGIN OF ENDOTHELIAL CELLS
The vascular system plays a vital homeostatic role in all vertebrates by promoting nutrient transport, oxygen, waste products and metabolites, immune surveillance, and the autoregulation of perfusion via chemical stimuli and hormones that help in the communication between the blood vessels and underlying tissues. Endothelial progenitor cells (EPCs) were discovered in the late 1990s resulting in a paradigm shift in our understanding of angiogenesis. The endothelium consists of a single layer of cells lining blood vessels in the body and is formed very early in gestation. Angiogenesis refers to the formation of new capillaries from existing vessels, while vasculogenesis refers to de novo formation of blood vessels during embryonic development.1–3 There are several sources of endothelial cells, including the neural crest cells, embryonic mesoderm, and hemangioblasts. These have been summarized in Figure 1.
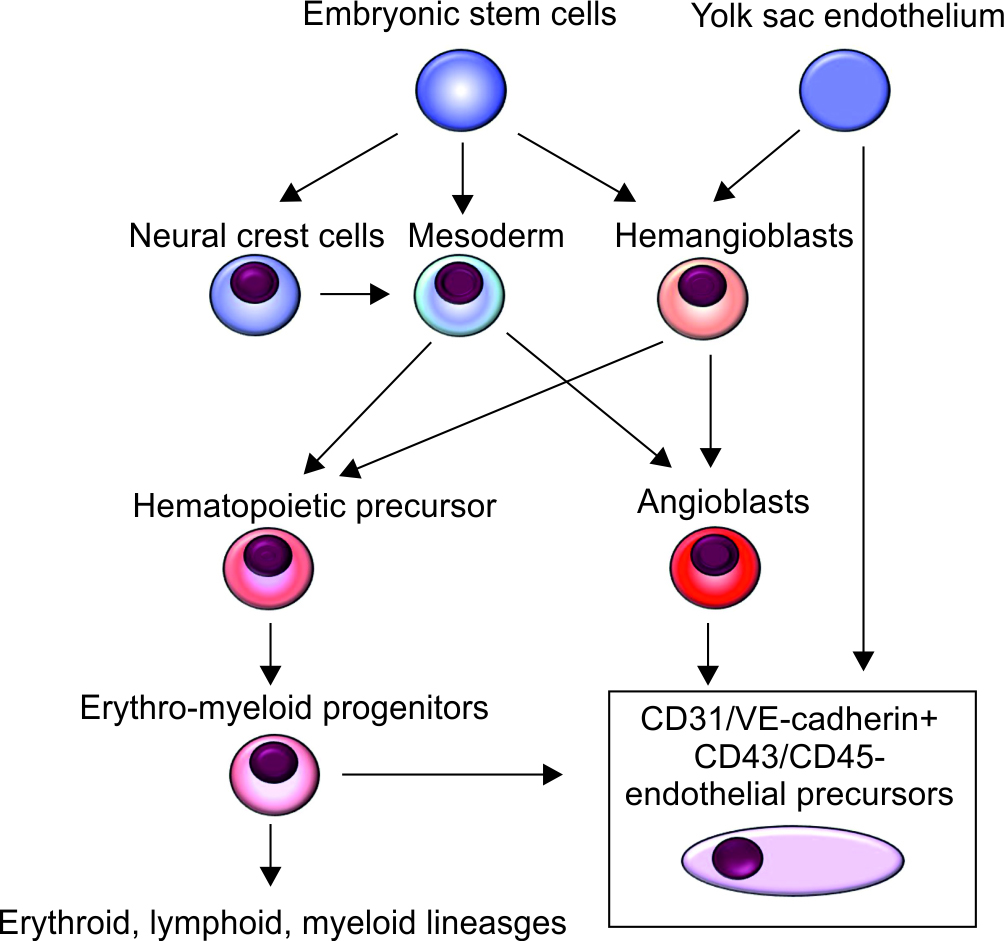
Fig. 1: Origin of endothelial cells. Overall schematic of the common origin of endothelial progenitor cells and the erythroid, lymphoid, myeloid precursors. Hematopoietic and endothelial progenitor cells are derived from a common precursor, the hemangioblast. Embryonic stem cells give rise to neural crest cells, mesoderm, and hemangioblasts. Hemangioblasts are derived from the yolk sac endothelium. Neural crest cells differentiate into mesenchymal stem cells, which tissue-resident precursors through chondro-, osteo- and adipogenesis. Endothelial precursors can arise from the yolk sac, myeloid precursors, and hemangioblasts
Differentiation from embryonic mesoderm: These primitive mesodermal cells differentiate into hematopoietic precursors or angioblasts, and these cellular subsets can both develop into endothelial cells.4,5 The intraembryonic endothelium forms a primitive vascular labyrinth2,6 shortly after gastrulation in the extraembryonic yolk sac. The endothelial/vascular maturation of mesodermal cells is induced by signals emanating from visceral endoderm,7 such as increased production of growth factors such as the basic fibroblast growth factor (bFGF) or FGF2, bone morphogenetic protein 4 (BMP4), and the vascular endothelial growth factor (VEGF).3,8
The transition of mesenchymal into endothelial cells may be a reversible, bidirectional process. The activation of transforming growth factor-β, bone morphogenetic protein, wingless/integrated (Wnt), and the Notch signaling pathways may be important in a mechanistic sense.9,10 The “dedifferentiation” and activation of endothelial cells involve a change in appearance from a characteristic cobblestone to a more elongated, “mesenchymal” shape with increased migratory and proliferative capacity. These transformed cells lose some of the intercellular junctional proteins and related barrier function7,10 but become pro-inflammatory with higher levels of leukocyte adhesion molecules (intercellular adhesion molecule 1, vascular cell adhesion molecule 1), cytokines, and various growth factors.11 However, with alterations in function, these changes may also shorten the lifespan of these cells. Such endothelial-to-mesenchymal transitions have been noted in various pathological conditions marked by vascular injury, chronic inflammation, and shear stress.10,11
Differentiation from hemangioblasts: Plein et al.12 showed that nearly a third of all endothelial cells in the brain and up to 60% of those in the liver may originate from hemangioblasts differentiating into erythro-myeloid progenitors (EMPs). These EMP-derived endothelial cells express high levels of the gene Hoxa. In another study, Feng et al.13 showed that these intraembryonic endothelial cells likely do not originate from circulating EMPs that express the cluster differentiation (CD) 45 (protein tyrosine phosphatase receptor type C)+. Csf1r-expressing EMPs may also not consistently differentiate into endothelial cells in the brain, liver, heart, and lungs.
The term “hemangioblast” was coined by Murray14 early in the 20th century to describe a subset of cells that can differentiate into either endothelial or hematopoietic cells during embryogenesis. This hypothesis found favor in the physical proximity of hematopoietic and endothelial lineages within blood islands,14,15 but the conclusions were not definitive due to the structural complexities in the developing blood islands and also because of the limited number of cells available to study during these early stages of development. Previous imaging and tissue engineering indicating spatiotemporal associations between these embryological hematopoietic and endothelial lineages16,17 and studies indicating that human embryonic stem cells are differentiated in vitro into both hematopoietic and endothelial cell lineages18 have largely been refuted.
Recent literature suggests that some of the hemogenic endothelium may be a source of hematopoietic stem cells (HSCs). Lineage-tracing studies, ex vivo culture, and time-lapse confocal imaging show that hematopoietic cells, including HSCs herald from a hemogenic endothelium, and form an intermediate endothelial state.4,19,20 Hemogenic endothelium is a specialized subset of the endothelium with only a transient capacity to produce hematopoietic cells through endothelial-to-hematopoietic transition.21 In murine models, endothelial cells that lose endothelial characteristics to assume a more hematopoietic phenotype begin to co-express surface markers CD144, CD31, KDR, CD117, and CD34, but not the hematopoietic markers, such as CD41, CD45, CD73, and Ter-119.21–24 Human hematogenic endothelial cells express the surface markers CD43, CD34, CD144, CD117, CD90, CD45, and CD105, but low CD38, and almost not CD45RA.22,25
The HSCs are self-renewing cells with multilineage reconstitution potential following transplantation into a recipient. After birth, HSCs are seen predominantly in the bone marrow and form a self-renewing pool at the apex of the hierarchical network of hematopoiesis. Some HSCs are also known to differentiate into hematopoietic progenitor cells (HPCs).22,26 The HPCs differ from HSCs with relatively limited self-renewal and engraftment potential.
Bone marrow-derived EPC: EPCs in the bone marrow have been redefined in numerous recent consensus statements to possibly originate from the following:
Endothelial Progenitor Cells: EPCs express surface markers, such as factor VIII, CD31, CD34, E-selectin (CD62E), intercellular adhesion molecule (ICAM)-1 (CD54), von Willebrand factor (vWF), and VCAM-1 (CD106).1,27–29 EPCs can migrate to the peripheral blood and express surface adhesion molecules that regulate the movement of these cells to and away from the blood.
Mesenchymal Stem Cells: Data on mesenchymal stem cells (MSCs) being a source of endothelial cells are controversial. Colony-forming units of fibroblasts (CFU-Fs) in the bone marrow, also known as the MSCs, express CD29, CD71, CD73, CD90, CD144, CD120a, CD105, CD106, and CD 124,30–33 but no CD34, CD31, vWF, vascular endothelium cadherin (VE-cadherin), VEGFR2, CD62E, VCAM-1, and ICAM-1.30–33 CD44 was detected in some studies,31,33 but not in others.30 MSCs expressing VEGFR2, vWF, and VE-cadherin are most likely endothelial progenitors.30,32,33 The discovery that mesenchymal cells can rescue damaged endothelial cells was demonstrated using laser scanning confocal microscopy to show that mitochondrial transfer was facilitated by a tunneling nanotube-like structure between human umbilical vein endothelial cells and MSCs.34
ENDOTHELIAL CELL PHENOTYPES
The phenotypic markers on endothelial cells can vary between various vascular structures in a particular organ and also between different organs. There may also be important structural variations notable within capillaries, veins, and arteries. The endothelium found in veins and arteries may seem to be comprised of an uninterrupted, continuous layer of cells; the capillary endothelium in various tissues can show more obvious differences and may appear continuous, discontinuous, or fenestrated.35 These spatial and temporal variations have been correlated with differential expression of various messenger RNA (mRNA) and proteins.
The first reported arterial EC marker was a transmembrane ligand ephrinB2 arterial.36 Notch signaling is vital to arterial EC differentiation. Loss of Notch signaling leads to a loss of the expression of ephrinB2 in the arteries in zebrafish.37 The first reported marker for venous EC was the ephrin B2 receptor tyrosine kinase EphB4.36 Venous EC differentiation is recognized as a default EC differentiation pathway, resulting from inadequate activation of Notch signaling during the differentiation of angioblasts to ECs.38,39 Lymphatic ECs are formed as a result of differentiation from venous ECs. Prox-1 is the most functional and specific EC of lymphatic origin. Disruption of mouse Prox-1 disrupts lymphatic vessel development and budding of lymphatic ECs.40 Insufficient activation of VEGFR3 signaling leads to hypoplastic lymphatic vessels.41
Endothelial cells have diverse microenvironments across vascular beds and display unique structural and morphologic heterogeneity across organs. The EC-translating ribosome affinity purification (TRAP) has emerged as a powerful tool to analyze the in vivo EC translatome across several diverse vascular beds to provide greater accuracy, sensitivity, and cellular resolution instead of whole-tissue RNASeq. TRAP identified 82 gene markers shared by five vascular beds (lung, heart, kidney, liver, and brain), such as Tek and pan-EC markers such as Eng, Nos3, Cdh5, and Robo4.42
Table 1 summarizes various endothelial cell markers; some are expressed after activation of growth factors and inflammatory cytokines, and others refer to specific endothelial cells in different organs or tissues. Endothelial cell phenotypes include the following:
Endothelial cells precursors: Embryonic ECs as a cell lineage expand without contribution circulating precursors or new angioblasts are the current consensus.12 The relationship of circulating endothelial progenitors to myeloid cells remains subject to controversy.43 Cells of myeloid origin are CD14+, while EPCs are CD14−. However, monocytes or macrophages (CD14+ cells) can adopt an endothelial phenotype during angiogenesis.44
Brain Endothelium: In the central nervous system, endothelial cells regulate plasma filtration and the movement of circulating cells through the blood-brain barrier, most likely via the assembly of tight junctions.45,46 Cerebral microvasculature likely originates from the meningeal vessels, but the subsequent angiogenesis involves the whole brain.47
| Type of marker | Name of marker | Species expressed | Cells expressed |
|---|---|---|---|
| Constitutive markers expressed in different endothelium | CD31/PECAM-1154 | Human, murine | Endothelial cells, B and T lymphocytes, platelets, monocytes, neutrophils |
| Bandeirea simplicifolia lectin binding155 | Murine | Endothelial cells | |
| Vascular endothelial cadherin39,156 | Human, murine | Endothelial cells, trophoblasts, macrophages | |
| CD3420 | Human, murine | Endothelial cells, hemopoietic precursors | |
| Thrombomodulin157 | Human, murine | Endothelial cells, smooth muscle cells | |
| Monoclonal antibodies used to identify specific endothelial cells | BMA-120158 | Human | ECs, mesothelium, glomerular epithelium |
| EN4158 | Human | Endothelial cells, leukocytes, platelets | |
| EN 7/44159 | Human | Endothelial cells in tumors and inflammatory tissues | |
| Endothelial cell markers induced by inflammatory cytokines | CD54/ICAM-122,160 | Human, murine | ECs, leukocytes, epithelium, fibroblasts |
| CD62E/E-selectin21 | Human, murine | Endothelial cells, postcapillary venules | |
| Endothelial cell markers induced by angiogenesis | KDR/Flk-1 (VEGFR-2)120,132,161 | Human, murine | Endothelial cells |
| Flt-1 (VEGFR-1)120,126,162 | Human, murine | Endothelial cells | |
| Tie-157,163 | Human, murine | Endothelial cells | |
| Tie-2/Tek57,163 | Human, murine | Endothelial cells |
HETEROGENEITY IN EPCS
EPCs are a heterogeneous population of mononuclear cells originating in the bone marrow and can be mobilized to the fetal/postnatal circulation.48–50 EPCs make up 1–5% of all bone marrow cells and about 0.0001–0.01% of monocytes circulating in the peripheral blood.51 These cells express endothelial antigens, like CD31, vWF, VE-cadherin, endothelial nitric oxide synthase (eNOS), and VEGFR2.52–55 The differentiation of hemangioblasts into endothelial cells has been studied in greater detail (Fig. 2). Based on phenotypical and biological properties, the EPCs are believed to be comprised of early and late EPC subgroups. Early EPCs give rise to the conventional colony-forming unit-endothelial cells (CFU-Es) and augment angiogenesis in a concentration-dependent or paracrine manner, whereas the outgrowth and differentiation of late EPCs promote the development of vascular networks.56 Early EPCs are spindle-shaped, CD133 + CD45 +, and have limited proliferative capacity, a relatively short lifespan of about 3–4 weeks, and secrete angiogenic factors, such as VEGF, interleukin-8, and the CXC-ligand 8/CXCL8. Late EPCs are cobblestone-shaped, CD31 + KDR +, appear at 2–3 weeks, may live up to 12 weeks, proliferate rapidly, and express VE-cadherin, Flt-1, and CD45.56,57 Both early and late EPCs seem to have comparable vasculogenic capacities.
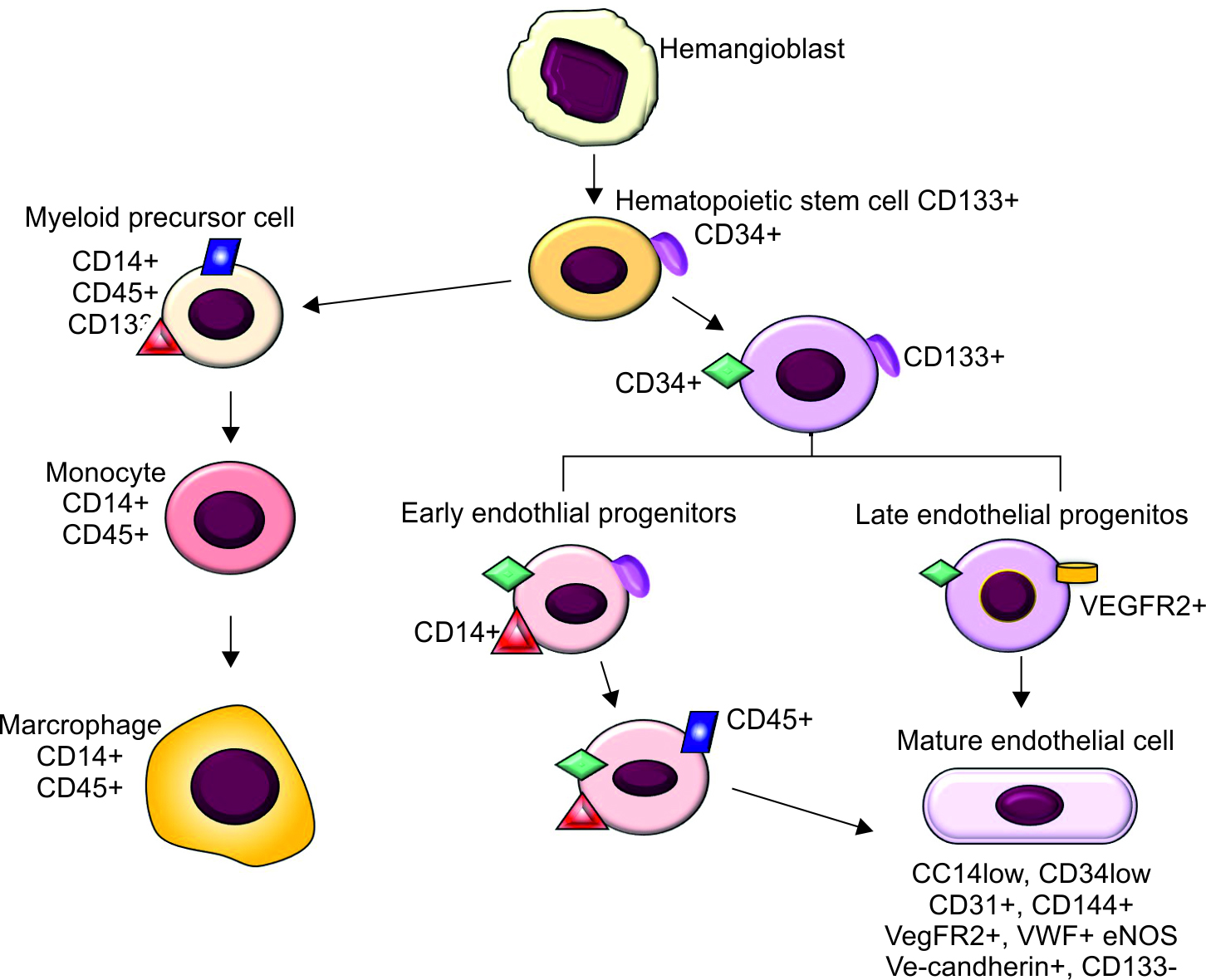
Fig. 2: Differentiation of endothelial progenitor cells. Hemangioblasts differentiate into hematopoietic stem cells and endothelial progenitor cells. Hematopoietic stem cells and endothelial progenitor cells express three markers cluster of differentiation (CD) 34, CD 45, CD133, and vascular endothelial growth factor receptor-2 (VEGFR2). CD133 is a marker for immature hematopoietic stem cell, while CD34 is a classic hematopoietic stem cell marker. Hematopoietic stem cells give rise to myeloid cell lineage, which express CD14 and CD45, and are CD133 negative, which ultimately give rise to monocytes and macrophages. As endothelial progenitor cells differentiate, they lose CD133 and begin to express CD31, CD144, vascular endothelial cadherin, VEGFR2, endothelial nitric oxide synthase (eNOS), and von Willebrand factor (vWF). Endothelial progenitor cells are positive for both hematopoietic stem cell marker CD34 or CD133 and an endothelial marker, such as VEGFR2. Endothelial progenitor cells do not have exclusive surface markers, rather share similar markers with mature endothelial cells
Based on gene expression profiles, endothelial cells increasingly seem to be a heterogeneous population. Endothelial subpopulations have been identified that show differences in the expression of bone morphogenic protein-2, -4; ephrin-4, and neuropilin-1. In the skin, distinct endothelial cells express platelet and endothelial cell adhesion molecule 1 (PECAM-1), notch-1, and leukocyte markers (ICAM-1, L-selectin, notch 2, CD36, and CD163).55 The aorta shows at least 3 distinct subpopulations, one comprised of lymphatic endothelial cells, whereas the other two seem to be specifically involved in angiogenesis, lipoprotein processing, and extracellular matrix production.58 The adult mouse lung contains a distinct subpopulation of endothelial cells that expresses high levels of carbonic anhydrase 4 (Car4) and is distinct from arterial and venous macrovascular, and microvascular endothelial cells.59 Car4-high endothelium is located throughout the lung periphery, expresses high levels of CD34 and VEGF receptors, and responds to VEGF-A. High numbers of Car4-high ECs can be seen in lung regions regenerating after influenza- or bleomycin-induced injury. The discovery of endothelial subsets with differing capacities for angiogenesis has opened exciting therapeutic possibilities.
ENDOTHELIAL CELL FUNCTION
Endothelial cells show a vast heterogeneity in function. The vascular endothelium is exposed to and responds to numerous tissue microenvironments, resulting in a substantial phenotypic heterogeneity in the vascular system. Epigenetic and non-epigenetic factors are responsible for determining this heterogeneity in the endothelium. Marcu et al.60 studied endothelial cells isolated from the lungs, heart, liver, and kidneys, and showed organ-specific ECs to have a unique expression of gene clusters, potential for angiogenesis, barrier properties, and metabolic rates, each of which enables their organ-specific functional and development properties. Endothelial cells are known to be highly ubiquitous and one of the most functionally diverse cell systems. Vascular endothelial lining regulates blood flow, nutrient delivery and waste removal; blood coagulation; inflammation; angiogenesis; and vascular remodeling through autonomous and intercellular signaling mediated via neurotransmitters, hormones, and cytokines; and interaction with several cells, such as smooth muscle cells, pericytes, cytokines, and blood cells.61 Prostacyclins and endothelium-derived nitric oxide (NO) cause vasodilation, while superoxide, endothelin, and thromboxane induce vasoconstriction; both sets of mediators regulate tissue perfusion.62
Endothelial Cell Barrier Function
The endothelial lining surface area is large and facilitates the substance exchange between blood and tissues. In humans, the endothelial surface area is estimated to be about 350 m2.63,64 Cells in the endothelial cell monolayer are linked to one another via tight, adherent, and gap junctions, which then connect to cytoplasmic proteins and the cytoskeleton.65 Interestingly, endothelial cells maintain a tight barrier function throughout the process of vascular remodeling; vasculogenesis stimulants, such as VEGF-A, do not change microvascular permeability in the inner blood-retina barrier in vivo or in vitro, even when specific changes may be seen in transcellular transport or in tight or adherens junctions.66–68
The plasma membranes of closely aligned endothelial cells form an important barrier with tight junctions. The main transmembrane constituent of these junctions is the occludins.69 Below the tight junctions, the adherens junctions are comprised of several proteins, including the surface adhesion glycoproteins, VE-cadherins, which form a zipper-like component at the base of endothelial cells. These proteins connect with their cytoplasmic tail to the underlying actin‐based microfilament cytoskeleton.70,71
Endothelial Cell Response to Shear Stress
Endothelial cells react actively to blood flow, predominantly to mechanical cues with polarizing changes in conformation, electrical charge, or to the release of biochemical stimuli, such as nitric oxide or prostacyclin.30,31,72 At rest, endothelial cells typically are shaped like a polygon, but under conditions of stress, they elongate in the direction of flow, thereby reducing the resistance to moving fluids.30 In response to shear stress, cultured endothelial cells elongate and become oriented along the direction of blood flow32 by reorganizing the cytoskeleton.33 Shear stress is known to directly activate the endothelial NO synthetase (eNOS) promoter and increase its expression, and also promote the release of endothelial cell factors that promote endothelial cell survival while inhibiting leukocyte migration, coagulation, and smooth muscle proliferation.30,72
Endothelial Cell as Regulator of Vascular Tone
Endothelial lining of vessels regulates vascular tone and function in response to numerous neurotransmitters, hormones, and vasoactive factors.62 The endothelium releases various vasoactive factors that can be vasodilatory, such as NO, prostacyclin (PGI2), and endothelium-derived hyperpolarizing factors (EDHF) or vasoconstrictive, such as thromboxane (TXA2) and endothelin-1(ET-1).62 Any imbalance of these vasoactive factors leads to dysfunction of the endothelium.
Endothelial Cells in Angiogenesis
The onset of neovascularization or angiogenic switch73 has several triggers, such as metabolic stress, hypoxia, inflammatory stimuli, and immune response, and may also be related to genetic mutations.74 During hypoxic conditions, hypoxia-responsive transcription factors regulate the expression of genes that allow tissues and cells to acclimatize to low oxygen conditions.74 VEGF as an endothelial cell-specific mitogen is unique for its roles in promoting endothelial cell proliferation and vascular permeability.49,75 VEGF can stimulate blood vessel development through the process of vasculogenesis or angiogenic sprouting, whereas ephrinB2 and Ang1 promote vascular remodeling and maturation of the vasculature.49,76 VEGF has three major isoforms that originate from alternative splicing, namely VEGF-A120, VEGF-A164, and VEGF-A188;77 these isoforms also exhibit anti- and pro-angiogenic splice variants. VEGF is known to have two transmembrane receptors, VEGFR1, otherwise known as the feline McDonough sarcoma (fms)-related receptor tyrosine kinase 1 (Flt1), and VEGFR2, otherwise known as the kinase insert domain receptor (Flk-1). VEGFR1 is known to be expressed either as a soluble Flt1 receptor (sFlt1) formed through alternative splicing of the Flt1 mRNA75 or as the membrane-bound Flt1. The two isoforms of VEGFR1 have a binding affinity that is tenfold higher for VEGF-A than VEGFR2.78 VEGF can prevent apoptosis in umbilical vein endothelial cells and human dermal microvascular endothelial by inhibiting the activity of stress-activated protein kinase/c-junNH2-kinase (SAPK/JNK) and activating the mitogen-activated protein kinase (MAPK) pathway.79
VEGF and Notch show synergistic effects to promote the formation of blood vessel branches. VEGFR2, not VEGFR1, stimulates the induction of tip cells and promotes vascular sprouting (Fig. 3).80 Notch is activated by the delta-like ligand 4 (DLL4) in neighboring endothelial cells; conversely, DLL4 inhibits tip cell behavior through the upregulation of VEGFR1 and the downregulation of VEGFR2 and VEGFR3 receptors.80,81 For effective angiogenesis, VEGF acts cooperatively with several factors, such as the angiopoietins (Ang).82 VEGF and Ang both have receptors on endothelial cells. Ang-1 and -2 bind to tyrosine kinase receptors, Tie 1 and Tie 283 (Fig. 3), while Ang-1, -2, and -4 all bind to the Tie 2 receptor.84 Ang-1 promotes vascular integrity by promoting endothelial cell migration, inhibiting endothelial cell apoptosis, promoting the generation of capillary-like structures, and recruiting pericytes to vascular tissues.84,85 Ang-1–Tie 2 signaling is shown to assist the maintenance of quiescent endothelial cell phenotype. Tie 2 interacts with the p85 subunit of phosphatidylinositol-3-kinase (PI3K) to activate the PI3K-AKT pathway, leading to increased survival and chemotaxis of endothelial cells.86,87 AKT activation inhibits the forkhead transcription factor FKHR (FOXO1), which may protect endothelial cells from apoptosis.88 Ang-1 and its binding to Tie 1 can promote vascular remodeling and are generally considered pro-angiogenic, whereas Ang-2 counteracts these effects and may be anti-angiogenic.89,90 Ang-2 is regarded as an agonist of Tie 2 and has been shown to stimulate Tie 2/Akt signaling, as well as inhibit the expression of FOXO1-target gene to enable the regulation of transcription and apoptosis. Ang-2 may also inhibit vascular permeability and acts as an autocrine agonist of Tie 2 and protect stressed endothelial cells.91

Fig. 3: Endothelial markers in inflammation and angiogenesis. VEGF works together with angiopoietins during inflammation and angiogenesis, and both have receptors on endothelial cells. Ang-1 and -2 bind to their receptors Tie 2. Ang-1–Tie 2 signaling contributes to maintaining a quiescent endothelial cell phenotype. Ang-1 is pro-angiogenic and required for vascular remodeling, while Ang-2 counteracts their effects as anti-angiogenic. VEGF has two transmembrane receptors, Flt1 or VEGFR1 and Flk-1 or VEGFR2. VEGFR1 has two forms generated by alternative splicing, a membrane-bound Flt1 and a soluble Flt1 receptor, VEGF signals through VEGFR2 to promote angiogenesis. VEGFR1 (Flt-1) serves to limit the actions of (VEGFR2) Flk-1. Ang-2 binds to Tie 2 to activate P13-K/Akt signaling. VEGF-VEGFR2 activates MAPK/AKT signaling pathways
In the brain, Ang-1 can be neuroprotective and inhibit apoptosis in brain neurons by activating phosphatidyl‐inositol 3‐kinase and also promoting the phosphorylation of Akt and restoring caspase-3 cleavage.92 Coadministration of VEGF-A and Ang-1 synergistically increased DNA synthesis, cell proliferation, endothelial cell migration, and sprouting more than either agent alone.93
Endothelial Cells and Inflammation
Endothelial cells can modulate the recruitment of inflammatory cells to locations of injury and produce cytokines, growth factors, colony‐stimulating factors, and chemokines in response to mechanical or chemical stimuli.52,94,95 These cytokines can then induce a feed-forward cycle by promoting cell–cell interactions and the proliferation and survival of endothelial cells and also by inducing an endothelial cell pro-inflammatory phenotype that produces cytokines [interleukin (IL)‐1], chemokines [IL‐8, monocyte chemoattractant protein (MCP)‐1], tumor necrosis factor (TNF), and adhesion molecules [vascular cell adhesion molecule (VCAM)‐1, intercellular adhesion molecule (ICAM)‐1, and endothelial (E)‐selectin], all of which recruit leukocytes to sites of injury.53,54 Activated endothelial cells recruit leukocytes to sites of infection, which is critical to host defense. Upregulation of related adhesion and ligands on these leukocytes by bacterial or host pro-inflammatory mediators promotes adherence to endothelial cells and focused migration to the sites of infection, where these cells may phagocytize and kill the pathogens.96
During inflammation, leukocytes migrate across the vascular endothelium into the tissues in a series of steps. The first steps involve relatively weak, adhesive interactions with the rapidly flowing leukocytes to slow these cells down, followed by a few halting, rolling tumbles on the endothelial surface. These interactions are gradually strengthened with the leukocyte activation and their subsequent adherence in the endothelium. These stationary leukocytes then migrate into the interstitium through spaces between adjacent endothelial cells. As one can imagine, this is an area of intense study. During transmigration across the vascular endothelium, leukocytes can take either the paracellular path to squeeze their way through between adjacent endothelial cells, or less frequently, show transcellular migration across individual endothelial cells.97 The principal endothelial adhesion molecules engaged in the attachment and transmigration of leukocytes include CD34, intercellular adhesion molecule 1 (ICAM1, CD54), endomucin (a membrane-bound glycoprotein expressed luminally by endothelial cells), ICAM2, the glycosylation-dependent cell adhesion molecule-1 (GLYCAM1), podocalyxin (a member of the sialomucin protein family), mucosal vascular addressin cell adhesion molecule 1 (MADCAM1), P-selectin, junctional adhesion molecule A (JAM-A), JAM-B, CD 99, vascular cell adhesion protein 1 (VAM1), CD106PECAM1, E-cadherin, and single-chain type-I glycoprotein.98–101
Endothelial Cells and Coagulation
Endothelial cells are important modulators of coagulation both in the physiological conditions and also during inflammation and infection. Endothelial cells express anticoagulant factors on their outer membrane surface. Loss of surface thrombin-binding proteins, such as thrombomodulin, and downstream protein C-mediated signaling play a vital role in minimizing thrombin activation and clotting in physiology. The loss of these factors leads to decreased ability of endothelial cells to modulate coagulation and inhibits the release of endothelium-derived factors, such as PGI2 and NO.102
ENDOTHELIAL CELLS IN NEONATAL DISORDERS
Fetal organs, especially the eye, lungs, and kidneys, show important vascular development in the third trimester of gestation. Therefore, impaired vascular development has been implicated in numerous conditions of prematurity, such as retinopathy of prematurity (ROP), bronchopulmonary dysplasia (BPD), and acute kidney injury (AKI). In neonates, endothelial cell function is well regulated in physiology and known to be altered in pathological states. Table 2 lists biomarkers of endothelial cell in various neonatal diseases.
| Neonatal disease | Biomarker | Functional properties | Functional use |
|---|---|---|---|
| IVH | IL-686 | Pro-inflammatory | Increased serum levels in IVH |
| IL-887 | Pro-inflammatory | Increased serum levels in IVH and white matter injury | |
| ROP | VEGF-A | Pro-angiogenic | Increased in ROP |
| sVEGFR-2120 | Pro-angiogenic | Elevated in premature infants with ROP | |
| sTie2120 | Pro-angiogenic | Elevated in premature infants with ROP | |
| IL-6118,119 | Pro-inflammatory | Increased amniotic fluid levels | |
| IL-8119 | Pro-inflammatory | Increased amniotic fluid levels | |
| NEC | PAF70,154,165 | Pro-inflammatory | Elevated in blood early in NEC |
| TGF-β153,166 | Pro-inflammatory | Increased blood levels in NEC | |
| Sepsis | Ang-181 | Anti-angiogenic | Decreased in sepsis |
| Ang-281 | Pro-angiogenic | Elevated in sepsis | |
| BPD | Ang-1128 | Anti-inflammatory | Reduced serum levels in BPD |
| Ang-2128 | Pro-inflammatory | Increased levels in BPD | |
| ICAM-1128 | Pro-inflammatory | Increased serum levels correlate with BPD severity | |
| IL-1β128 | Pro-inflammatory | Increased serum levels in infants with both BPD and pulmonary hypertension. | |
| MCP-1128 | Pro-inflammatory | Lower serum levels of MCP-1 | |
| VEGF126 | Pro-angiogenic | Decreased levels in BPD | |
| Tie 2126 | Pro-angiogenic | Decreased levels in BPD |
Endothelial Cells in Neonatal Sepsis
The incidence of early-onset neonatal sepsis with positive cultures in newborns is about 0.98/1,000 live births and most likely higher in very-low-birth-weight (VLBW) infants.103 The incidence of late-onset sepsis is more variable and could be as high as 30% in extremely low-birth-weight (ELBW) infants.104 A dysregulated immune host response is associated with the pathogenesis of neonatal sepsis. Gram-negative sepsis has high mortality rates, with most mortality occurring in the acute phase, within the first three days of onset of sepsis.105
During inflammation, the vascular endothelium expresses a plethora of cytokines with a local chemotactic gradient that recruits the leukocytes into peripheral tissues. Such recruitment responses in neonates may be weaker in most organs when compared to adults and in preterm in comparison to term neonates.106,107 In other organs such as the intestine, particularly during necrotizing enterocolitis, the recruitment may be enhanced. Inflammation of vascular endothelium during sepsis leads to altered chemotaxis and leukocyte transmigration because of the impaired endothelial expression of adhesion molecules, such as E-selectin, ICAM-1, and P-selectin.108 Some of these changes may be related to altered expression of pro-inflammatory ligands such as TNF, which can affect the expression of adhesion molecules VCAM-1 and ICAM-1.29
Biomarkers that regulate endothelial cells and reflect their microenvironment may be useful in monitoring sepsis. Angiopoietins stimulate endothelial cells to increase or suppress inflammation. Ang-1 expressed in peri-endothelial cells can suppress inflammatory responses and stabilize the microvasculature by inhibiting nuclear factor κB (NFκB) activation. In contrast, Ang-2, which is expressed preferentially in endothelial cells, is pro-inflammatory and can increase the permeability of vessels and destabilize them. Ang-1 binds to Tie 2, the tyrosine kinase receptor to maintain the endothelial resting state, thereby suppressing vascular permeability during inflammation (Fig. 3).109
Endothelial Cells in the Neonatal Brain
Endothelial cells in the brain microvasculature have an intricate relationship with neuronal development and function, suggestive of a neurovascular crosstalk. Endothelial cells stimulate the proliferation and differentiation of neuronal precursors toward neuronal lineage.50 During postnatal development, endothelial cells promote excitatory synaptogenesis through upregulation of VEGF expression in cortical neurons by increased signaling through the P38/MAPK pathway.110 The premature infant is sensitive to neurologic injury partly due to the exposure of their immature vascular network to extrauterine physiologic abnormalities in oxygen tension, biochemical, and environmental factors.
Intraventricular hemorrhage occurs in about 20% of infants born before 32 weeks’ gestation and is a major cause of neurodevelopmental morbidity and mortality in premature infants.111,112 IL-6 may be an important early biomarker for IVH. In one study, serum IL-6 levels were elevated in infants with IVH and were associated with increased risk of neonatal morbidity at less than 28 days after birth.113 If high levels of IL-8, an important neutrophil and monocyte chemokine that is produced by macrophages, smooth muscle cells, and the endothelium, persisted for >1 day, the risk of IVH and white matter injury was higher.114
Hypoxic-ischemic encephalopathy (HIE) is a encephalopathy resulting from perinatal asphyxia that leads to neuronal death from activation of inflammatory cells and overexpression of apoptosis-related proteins.115 In HIE, elevated inflammatory cytokine levels such as IL-6, TNF, and IL-8 recruit leukocytes to the site of injury and damage endothelial cell integrity.116,117 In infants with HIE, early microvascular injury may have a critical impact on neuronal damage.118
Endothelial Cells in Retinopathy of Prematurity
Premature infants continue to develop the retinal vasculature after birth and are susceptible to altered vascular development such as in retinopathy of prematurity (ROP). These abnormalities in angiogenesis can be recapitulated in murine models such as those of oxygen-induced retinopathy (OIR).119–121 ROP involves altered endothelial cell proliferation and survival,122–124 and consequent abnormal retinal vascularization. Increasing data suggest that ROP involves dysregulation of VEGF expression.125,126 The vascular development in ROP shows two distinct phases: an initial phase of vaso-obliteration that is triggered by hypoxia and a subsequent period of abnormal neovascularization triggered by retinal hypoxia to meet the demands of the metabolically hyperactive retinal cells and neurons.127 These abnormalities can be seen in the mouse model of OIR, where mouse pups exposed first to hyperoxia develop vaso-obliteration of retinal vessels and then show abnormal neovascularization.128,129
In vivo assessments in a mouse oxygen-induced retinopathy model have revealed several physiologic and functional phenotypes in the developing retinal as a result of aberrant angiogenesis. Alterations in arterial and venous oxygen tension (PO2) result in increased arterio-venous PO2 gradients, which indicate increased oxygen extraction and possible underlying ischemia.130 Whole-mount staining of retinas shows central vaso-obliteration in neonatal OIR mice with recovery to full vascularization by P21.120,128,129 However, longitudinal live retinal imaging using fluorescein angiography revealed capillary avascularity, arterial tortuosity, and venous dilation in neonatal OIR mice compared to fully vascularized, normal caliber arteries and veins in room-air-raised mice, and consequent prolonged loss of capillary density with the paucity of neovascular buds on capillaries of adult OIR mice in spite of full peripheral vascularization. Spectral-domain optical coherence tomography revealed thinner retinas in neonatal mice with OIR,131–134 more pronounced in the hypovascular retinal areas,132 and restricted to the inner retina.133 Electroretinograms correlate retinal vascular abnormalities to inner retinal dysfunction in OIR mice.134,135 Comparative retinal histology following in vivo imaging showed prolonged over-expression of VEGF, microglial activation, abnormal malaligned neuronal synapses, and apoptosis in OIR mice.136 A subpopulation of resident macrophages (M2) has been shown to be an important phenotype during angiogenesis.137,138 Exogenous administration of pro-angiogenic isoform of VEGFA165a in a mouse model of OIR promoted earlier revascularization,126 likely by targeting endothelial cell proliferation via increased angiogenic signaling through VEGF receptors.
Several proteins and support cells are intricately linked to endothelial cell function. Endothelial cells have surface protein receptors for integrins that play a role in angiogenesis and inflammation.139 VEGF induces expression of the collagen receptors, α1β1 and α2β1 integrins.140 The recruitment of pericytes has been demonstrated to be important in vascular maturation, for stabilization of the vasculature and remodeling of the early endothelial plexus into a more mature vascular network.141,142 Disruption of endothelial-pericyte connections leads to exaggerated regression of vasculature and abnormal remodeling.141 Angiopoietins play a role in the pathogenesis of ROP. Ang-2 was inhibited by hyperoxia and increased during relative hypoxia in a rat model of OIR.143 Biomarkers have been investigated for ROP monitoring and disease severity. IL-6 levels in the umbilical cord were noted to be elevated in preterm infants with severe ROP, while high cord levels plasma C5a were associated with ROP that required laser therapy.144
Inflammatory cytokines have been associated with ROP in both the peri- and postnatal periods. Studies of amniotic fluid samples from 175 premature infants born between 23–32 weeks showed that higher IL-6 and IL-8 levels were associated with a higher risk of advanced ROP.145 Similarly, Pieh et al. showed that premature infants with high plasma levels of the soluble VEGF receptor 2 (sVEGFR-2) and its soluble membrane-bound tyrosine kinase receptor (sTie) are associated with an increased risk of ROP.146
Endothelial Cells in Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD), also known as chronic lung disease of prematurity, is related to increased supplemental oxygen use during the early neonatal period147,148 and occurs in about 40% of infants born below 29 weeks gestation.149,150 A coordinated development of the pulmonary vasculature is required for normal lung development growth. Preterm birth may disrupt the lung vascular growth during the saccular and alveolar stages of pulmonary development, and aberrant development of the pulmonary vascular bed may lead to impaired alveolar development.148,150,151 Postmortem lung examination of infants with BPD showed low levels of VEGF mRNA and reduced VEGF immunostaining, as well as a reduction in angiogenic receptors Flt-1 and Tie 2 in the infants with BPD compared to those without BPD.152 Inhibiting VEGF during development decreases alveolarization and pulmonary arterial density.153,154 Higher levels of ICAM-1, Ang-2, and IL-1β, and reduced levels of Ang-1 and MCP-1 are correlated with BPD severity.155
Endothelial Cells in Pulmonary Hypertension
Endothelial dysfunction is centrally implicated in pulmonary hypertension. Pulmonary hypertension is a multifactorial and complex condition, associated with the aberrant endothelial cell proliferation with concurrent neoangiogenesis and the alteration in the secretion of vasoactive mediators, such as prostacyclin, NO, serotonin, ET-1, and thromboxane. The lung endothelium is heterogenous and different from systemic endothelium in both function and structure. The pulmonary endothelium’s function includes maintaining barrier integrity, homeostasis, vascular tone, leukocyte trafficking, and production of necessary growth factors.156 The normal endothelium is typically in a stable, “quiescent” state. When the endothelium is disturbed and “activated” by stress, infection, disease, or injury, endothelial cells tend to express specific proteins and markers, such as ICAM-1, VEGF, and E-selectin, which causes exaggerated proliferation, coagulability, and vasoconstriction.156,157 In pulmonary hypertension, some of the triggers of endothelial activation are inflammation, shear stress, reactive oxygen species, genetic mutations, and defect in angiogenesis.156
Endothelial Cells in Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) is an inflammatory bowel disease seen in premature infants that is associated with high morbidity and mortality. Maldevelopment of the microvasculature of the intestinal mucosal and abnormally altered intestinal blood flow are implicated in the pathogenesis of NEC. There is low resistance of the intestinal vasculature across the intestines of the newborn infant, mediated by increase in the production of nitric oxide by the endothelium. Neonatal swine models showed abnormal vasoconstriction responses to severe hypoxemia, resulting in intestinal ischemia.158 Hypoxia in the preterm neonate can inhibit NO production and result in intestinal injury and NEC.159 There is also evidence of VEGF dysregulation; premature infants exposed to hyperoxia may show decreased VEGF expression and VEGF/VEGFR-regulated pro-angiogenic signaling pathways and diminished development of the intestinal microvasculature. These limitations in the splanchnic vasculature may not be insufficient for the relatively limited metabolic needs in the first few days after birth but may become inadequate with increasing feeding volumes in the later neonatal period. In experimental NEC, VEGFR2 protein and VEGFR2 activity have been shown to be low preceding the onset of intestinal injury.160,161 Similarly, inhibition of VEGFR2 led to decreased endothelial cell proliferation and intestinal microvascular network development.161 Administration of dimethyloxalylglycine (DMOG), a propyl hydroxylase enzyme inhibitor, increased the expression of VEGF-A in the intestines of neonatal pups, but the splanchnic effects of DMOG were abolished by inhibiting VEGFR2 signaling.162 Further investigations are needed to investigate the strategies to modulate angiogenic signaling through the VEGF-VEGFR2 pathway, which may possibly protect against NEC.
Endothelial Cells in Neonatal Acute Kidney Injury
Early changes in capillary blood flow and endothelial cell injury leading to inflammation, ischemia, and pro-coagulation may play a crucial role in the pathogenesis of early and chronic ischemic AKI. In rat models, ischemic kidneys were unable to autoregulate blood flow and exhibited vasoconstriction when renal perfusion pressure decreased.163 The organization of the cytoskeletal network of endothelial cells and small arterioles is altered during renal ischemia-reperfusion injury, which disrupts endothelial cell tight junctions as indicated by the disintegration of VE-cadherin in renal microvasculature.164,165 The loss of the integrity of barrier function could have been the result of matrix metalloproteinase-2 or -9 activation.166 There is also some evidence to show impaired endothelial-dependent vasodilator activity in AKI. L-arginine and eNOS cofactor tetrahydrobiopterin may attenuate acute ischemia-reperfusion renal injury by preserving medullary perfusion.167–169
ENDOTHELIAL CELLS AS THERAPEUTIC TARGETS
Therapeutic advances to regulate angiogenesis have been challenging and limited in success employing pro- and anti-angiogenic factors. This could be due to the complex biology of angiogenic factors, their multiple receptors, and versatile functions. Preclinical studies of pro-angiogenic cell therapies or microRNAs targeting show promise of alternate therapeutic strategies.170
Bevacizumab (Avastin) is a promising non-selective anti-VEGF drug that was first used to treat metastatic cancers171 but was subsequently approved for the treatment of ROP and other ocular conditions.171–173 Selective pro-angiogenic VEGF isoforms are being explored preclinically, such as administration of VEGFA165a microparticles for the treatment of ROP.126 Ranibizumab, a humanized Fab fragment that can block all VEGF isoforms, reverses VEGF-stimulated delocalized tight junctions, proliferation and migration of cells, and delocalization of tight junction proteins in retinal endothelial cells, may also be useful in some stages of ROP.68 Targeting endothelial-to mesenchyme transitions may also be useful in specific stages of vascular disease. Relaxin, a calcimimetic agent, Cinacalcet, and Losartan are shown to inhibit endothelial-mesenchymal transitions.174–176
There may be some utility in monitoring biomarkers indicative of damage to the endothelium during neonatal sepsis, such as endothelial growth factors or components of tight junctions (TJs) that shed into circulation upon endothelial damage and quantifying plasma and urine levels of soluble components of endothelial wall and glycocalyx and degraded glycocalyx.177 Soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) is able to differentiate sepsis from non-sepsis cases, with an area under curve (AUC) of 0.97 to diagnose proven or suspected neonatal sepsis, compared to 0.96 of IL-6, and 0.8 of Endocan.178 The ratio of Ang-1 is shown to correlate with bacteremia.179 Higher Ang-2 levels correlate with clinical sepsis.180 Endothelial cell dysfunction has also been implicated in NEC, and several therapies are being explored to modulate the ensuing inflammatory necrosis. Enteral administration of TGF-β2 was protective in mice with experimental NEC-like injury.181 PAF has been implicated in NEC pathogenesis and shows promise as a biomarker.182 Resveratrol (trans-3,4′,5-trihydroxystilbene) is a naturally occurring polyphenol found in red wine, berries, and peanuts and has been shown to improve endothelial NO production and endothelial redox balance, as well as inhibit the activation of the endothelium following pro-inflammatory and metabolic stress.183 Protocols have been developed that enable the differentiation of h-iPSCs very efficiently into competent h-iECs, thereby enabling the development of perfused vascular networks in vivo.184 Despite the early promises of tissue engineering involving endothelial cells, applications to clinical practice are limited. Understanding the cellular and molecular mechanisms related to physiologic and pathologic angiogenesis, both in pediatric and adult tissues, will enhance advances in tissue engineering.185
CONCLUSION
Endothelial cells are critical regulators of vascular homeostasis through intricate interactions with vascular smooth muscle cells, circulating cells, and surrounding support cells, and their connections to blood and tissue components make them vulnerable to minute alterations in the composition of blood, mechanical stress of blood flow, injury, or inflammation. Endothelium based on the microenvironment can transform from pro-inflammatory to anti-inflammatory properties, as well as vasodilation or vasoconstriction, and pro- and anti-thrombotic properties. Future investigations focused on understanding endothelial cell heterogeneity may provide insights into vascular-bed-specific therapies in neonates.
ORCID
Akhil Maheshwari https://orcid.org/0000-0003-3613-4054
REFERENCES
1. Chopra H, Hung MK, Kwong DL, et al. Insights into endothelial progenitor cells: origin, classification, potentials, and prospects. Stem Cells Int 2018:9847015. DOI: 10.1155/2018/9847015.
2. Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol 1995;11:73–91. DOI: 10.1146/annurev.cb.11.110195.000445.
3. Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res 2000;55:15–35. PMID: 11036931.
4. Zovein AC, Hofmann JJ, Lynch M, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 2008;3(6):625–636. DOI: 10.1016/j.stem.2008.09.018.
5. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011;146(6):873–887. DOI: 10.1016/j.cell.2011.08.039.
6. Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood 2000;95(5):1671–1679. PMID: 10688823.
7. Dejana E, Hirschi KK, Simons M. The molecular basis of endothelial cell plasticity. Nat Commun 2017;8(1):1–11. DOI: 10.1038/ncomms14361.
8. Marcelo KL, Goldie LC, Hirschi KK. Regulation of endothelial cell differentiation and specification. Circulation Res 2013;112(9):1272–1287. DOI: 10.1161/CIRCRESAHA.113.300506.
9. Lu X, Gong J, Dennery PA, et al. Endothelial-to-mesenchymal transition: Pathogenesis and therapeutic targets for chronic pulmonary and vascular diseases. Biochem Pharmacol 2019;168:100–107. DOI: 10.1016/j.bcp.2019.06.021.
10. Chen PY, Schwartz MA, Simons M. Endothelial-to-mesenchymal transition, vascular inflammation, and atherosclerosis. Front Cardiovasc Med 2020;7:53. DOI: 10.3389/fcvm.2020.00053.
11. Schwartz MA, Vestweber D, Simons M. A unifying concept in vascular health and disease. Science 2018;360(6386):270–271. DOI: 10.1126/science.aat3470.
12. Plein A, Fantin A, Denti L, et al. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature 2018;562(7726):223–228. DOI: 10.1038/s41586-018-0552-x.
13. Feng T, Gao Z, Kou S, et al. No evidence for erythro-myeloid progenitor-derived vascular endothelial cells in multiple organs. Circ Res 2020;127(10):1221–1232. DOI: 10.1161/CIRCRESAHA.120.317442.
14. Murray PDF. The development in vitro of the blood of the early chick embryo. Proc R Soc London Ser B 1932;111(773):497–521. DOI: 10.1098/rspb.1932.0070.
15. Sabin FR. Studies on the origin of blood-vessels and of red blood-corpuscles as seen in the living blastoderm of chicks during the second day of incubation. In: Contributions to embryology. vol. 9. Carneg Inst.; 1920. p. 214–262.
16. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292(5819):154–156. DOI: 10.1038/292154a0.
17. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci 1981;78(12):7634–7638. DOI: 10.1073/pnas.78.12.7634.
18. Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci 2001;98(19):10716–10721. DOI: 10.1073/pnas.191362598.
19. Fraser ST, Ogawaa M, Yu RT, et al. Definitive hematopoietic commitment within the embryonic vascular endothelial-cadherin+ population. Exp Hematol 2002;30(9):1070–1078. DOI: 10.1016/S0301-472X(02)00887-1.
20. Boisset JC, van Cappellen W, Andrieu-Soler C, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 2010;464(7285):116–120. DOI: 10.1038/nature08764.
21. Kissa, K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 2010;464(7285):112–115. DOI: 10.1038/nature08761.
22. Lange L, Morgan M, Schambach A. The hemogenic endothelium: a critical source for the generation of PSC-derived hematopoietic stem and progenitor cells. Cell Mol Life Sci 2021;78(9):4143–4160. DOI: 10.1007/s00018-021-03777-y.
23. Nadin BM, Goodell MA, Hirschi KK. Phenotype and hematopoietic potential of side population cells throughout embryonic development. Blood 2003;102(7):2436–2443. DOI: 10.1182/blood-2003-01-0118.
24. Eilken HM, Nishikawa SI, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 2009;457(7231):896–900. DOI: 10.1038/nature07760.
25. Ivanovs A, Rybtsov S, Anderson RA, et al. Identification of the niche and phenotype of the first human hematopoietic stem cells. Stem Cell Rep 2014;2(4):449–456. DOI: 10.1016/j.stemcr.2014.02.004.
26. Appelbaum FR. Hematopoietic-cell transplantation at 50. New Engl J Med 2007;357(15):1472. DOI: 10.1056/NEJMp078166.
27. Krause DS, Fackler MJ, Civin CI, et al. CD34: structure, biology, and clinical utility. Blood 1996;87(1):1–13. PMID: 8547630.
28. Kansas GS. Selectins and their ligands: current concepts and controversies. Blood 1996;88(9):3259–3287. PMID: 8896391.
29. Qureshi MH, Cook-Mills J, Doherty DE, et al. TNF-α-dependent ICAM-1-and VCAM-1-mediated inflammatory responses are delayed in neonatal mice infected with Pneumocystis carinii. J Immunol 2003;171(9):4700–4707. DOI: 10.4049/jimmunol.171.9.4700.
30. Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci 1996;109(4):713–726. DOI: 10.1242/jcs.109.4.713.
31. Reinhart W. Shear-dependence of endothelial functions. Experientia 1994;50(2):87–93. DOI: 10.1007/BF01984940.
32. Levesque M, Nerem R. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng 1985;107(4):341. DOI: 10.1115/1.3138567.
33. Noria S, Xu F, McCue S, et al. Assembly and reorientation of stress fibers drives morphological changes to endothelial cells exposed to shear stress. Am J Pathol 2004;164(4):1211–1223. DOI: 10.1016/S0002-9440(10)63209-9.
34. Liu K, Ji K, Guo L, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvascular Res 2014;92:10–18. DOI: 10.1016/j.mvr.2014.01.008.
35. Aird WC. Endothelial cell heterogeneity. Cold Spring Harbor Perspect Med 2012;2(1):a006429. DOI: 10.1101/cshperspect.a006429.
36. Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 1998;93(5):741–753. DOI: 10.1016/s0092-8674(00)81436-1.
37. Lawson ND, Weinstein BM. Arteries and veins: making a difference with zebrafish. Nat Rev Genet 2002;3(9):674–682. DOI: 10.1038/nrg888.
38. Thurston G, Yancopoulos GD. Gridlock in the blood. Nature 2001;414(6860):163–164. DOI: 10.1038/35102664.
39. Yamashita JK. Differentiation of arterial, venous, and lymphatic endothelial cells from vascular progenitors. Trends Cardiovasc Med 2007;17(2):59–63. DOI: 10.1016/j.tcm.2007.01.001.
40. Wigle JT, Harvey N, Detmar M, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 2002;21(7):1505–1513. DOI: 10.1093/emboj/21.7.1505.
41. Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 2004;5(1):74–80. DOI: 10.1038/ni1013.
42. Cleuren AC, van der Ent MA, Jiang H, et al. The in vivo endothelial cell translatome is highly heterogeneous across vascular beds. Proc Natl Acad Sci 2019;116(47):23618–23624. DOI: 10.1073/pnas.1912409116.
43. Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 2008;28(9):1584–1595. DOI: 10.1161/ATVBAHA.107.155960.
44. Pujol BF, Lucibello FC, Gehling UM, et al. Endothelial-like cells derived from human CD14 positive monocytes. Differentiation 2000;65(5):287–300. DOI: 10.1046/j.1432-0436.2000.6550287.x.
45. Garlanda C, Dejana E. Heterogeneity of endothelial cells: specific markers. Arterioscler Thromb Vasc Biol 1997;17(7):1193–1202. DOI: 10.1161/01.atv.17.7.1193.
46. Wolburg H, Neuhaus J, Kniesel U, et al. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci 1994;107(5):1347–1357. DOI: 10.1242/jcs.107.5.1347.
47. Risau W, Wolburg H, Development of the blood-brain barrier. Trends Neurosci 1990;13(5):174–178. DOI: 10.1016/0166-2236(90)90043-a.
48. Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275(5302):964–966. DOI: 10.1126/science.275.5302.964.
49. Michiels C. Endothelial cell functions. J Cell Physiol 2003;196(3):430–443. DOI: 10.1002/jcp.10333.
50. Sosa MAG, Gasperi RD, Rocher AB, et al. Interactions of primary neuroepithelial progenitor and brain endothelial cells: distinct effect on neural progenitor maintenance and differentiation by soluble factors and direct contact. Cell Res 2007;17(7):619–626. DOI: 10.1038/cr.2007.53.
51. Khan SS, Solomon MA, McCoy JP Jr. Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytomet Part B Clin Cytomet J Int Soc Anal Cytol 2005;64(1):1–8. DOI: 10.1002/cyto.b.20040.
52. Krishnaswamy G, Kelley J, Yerra L, et al. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J Interferon Cytokine Res 1999;19(2):91–104. DOI: 10.1089/107999099314234.
53. Bussolino F, Camussi G, Tetta C, et al. Selected cytokines promote the synthesis of platelet-activating factor in vascular endothelial cells: comparison between tumor necrosis factor alpha and beta and interleukin–1. J Lipid Mediat 1990;2:S15–S22. PMID: 2133280.
54. Collins T, Read MA, Neish AS, et al. Transcriptional regulation of endothelial cell adhesion molecules: NF‐κB and cytokine‐inducible enhancers. FASEB J 1995;9(10):899–909. PMID: 7542214.
55. Abdelgawad ME, Desterke C, Uzan G, et al. Single-cell transcriptomic profiling and characterization of endothelial progenitor cells: new approach for finding novel markers. Stem Cell Res Ther 2021;12(1):145. DOI: 10.1186/s13287-021-02185-0.
56. Sieveking DP, Buckle A, Celermajer DS, et al. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol 2008;51(6):660–668. DOI: 10.1016/j.jacc.2007.09.059.
57. Du X, Hu N, Yu H, et al. miR-150 regulates endothelial progenitor cell differentiation via Akt and promotes thrombus resolution. Stem Cell Res Ther 2020;11(1):1–13. DOI: 10.1186/s13287-020-01871-9.
58. Kalluri AS, Vellarikkal SK, Edelman ER, et al. Single-cell analysis of the normal mouse aorta reveals functionally distinct endothelial cell populations. Circulation 2019;140(2):147–163. DOI: 10.1161/CIRCULATIONAHA.118.038362.
59. Niethamer TK, Stabler CT, Leach CT, et al. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. Elife 2020;9:e53072. DOI: 10.7554/eLife.53072.
60. Marcu R, Choi YJ, Xue J, et al. Human organ-specific endothelial cell heterogeneity. IScience 2018;4:20–35. DOI: 10.1016/j.isci.2018.05.003.
61. McCarron JG, Wilson C, Heathcote HR, et al. Heterogeneity and emergent behaviour in the vascular endothelium. Curr Opin Pharmacol 2019;45:23–32. DOI: 10.1016/j.coph.2019.03.008.
62. Sandoo A, van Zanten JJCSV, Metsios GS, et al. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J 2010;4:302. DOI: 10.2174/1874192401004010302.
63. Baldwin AL, Thurston G. Mechanics of endothelial cell architecture and vascular permeability. Crit Rev Biomed Eng 2001;29(2):247. DOI: 10.1615/critrevbiomedeng.v29.i2.20.
64. Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflügers Archiv 2000;440(5):653–666. DOI: 10.1007/s004240000307.
65. Dejana E, Corada M, Lampugnani MG. Endothelial cell‐to‐cell junctions. FASEB J 1995;9(10):910–918. PMID: 7615160.
66. Antonetti DA, Barber AJ, Hollinger LA, et al. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1 A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem 1999;274(33):23463–23467. DOI: 10.1074/jbc.274.33.23463.
67. Suarez S, McCollum GW, Bretz CA, et al. Modulation of VEGF-induced retinal vascular permeability by peroxisome proliferator-activated receptor-β/δ. Invest Ophthalmol Vis Sci 2014;55(12):8232–8240. DOI: 10.1167/iovs.14-14217.
68. Deissler H, Lang S, Lang GE. VEGF-induced effects on proliferation, migration and tight junctions are restored by ranibizumab (Lucentis) in microvascular retinal endothelial cells. Br J Ophthalmol 2008;92(6):839–843. DOI: 10.1136/bjo.2007.135640.
69. Bazzoni G, Estrada OMMN, Dejana E. Molecular structure and functional role of vascular tight junctions. Trends Cardiovasc Med 1999;9(6):147–152. DOI: 10.1016/s1050-1738(99)00022-5.
70. Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest 1996;98(9):1949–1953. DOI: 10.1172/JCI118997.
71. Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 2008;121(13):2115–2122. DOI: 10.1242/jcs.017897.
72. Xiao Z, Zhang Z, Diamond SL. Shear stress induction of the endothelial nitric oxide synthase gene is calcium‐dependent but not calcium‐activated. J Cell Physiol 1997;171(2):205–211. PMID: 9130468.
73. Folkman J. Tumor angiogenesis: therapeutic implications. New Engl J Med 1971;285(21):1182–1186. DOI: 10.1056/NEJM197111182852108.
74. Dachs G, Tozer G. Hypoxia modulated gene expression: angiogenesis, metastasis and therapeutic exploitation. Eur J Cancer 2000;36(13):1649–1660. DOI: 10.1016/s0959-8049(00)00159-3.
75. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9(6):669–676. DOI: 10.1038/nm0603-669.
76. Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature 2000;407(6801):242–248. DOI: 10.1038/35025215.
77. Vempati P, Popel AS, Mac Gabhann F. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev 2014;25(1):1–19. DOI: 10.1016/j.cytogfr.2013.11.002.
78. Waltenberger J, Claesson-Welsh L, Siegbahn A, et al. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 1994;269(43):26988–26995. PMID: 7929439.
79. Gupta K, Kshirsagar S, Li W, et al. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res 1999;247(2):495–504. DOI: 10.1006/excr.1998.4359.
80. Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell 2009;16(2):196–208. DOI: 10.1016/j.devcel.2009.01.015.
81. Jakobsson L, Franco CA, Bentley K, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol 2010;12(10):943–953. DOI: 10.1038/ncb2103.
82. Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999;284(5422):1994–1998. DOI: 10.1126/science.284.5422.1994.
83. Sato TN, Qin Y, Kozak CA, et al. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci 1993;90(20):9355–9358. DOI: 10.1073/pnas.90.20.9355.
84. Jones N, Iljin K, Dumont DJ, et al. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol 2001;2(4):257–267. DOI: 10.1038/35067005.
85. Witzenbichler B, Maisonpierre PC, Jones P, et al. Chemotactic properties of angiopoietin-1 and-2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem 1998;273(29):18514–18521. DOI: 10.1074/jbc.273.29.18514.
86. Brkovic A, Pelletier M, Girard D, et al. Angiopoietin chemotactic activities on neutrophils are regulated by PI‐3K activation. J Leukocyte Biol 2007;81(4):1093–1101. DOI: 10.1189/jlb.0906580.
87. Harfouche R, Hasséssian HM, Guo Y, et al. Mechanisms which mediate the antiapoptotic effects of angiopoietin-1 on endothelial cells. Microvasc Res 2002;64(1):135–147. DOI: 10.1006/mvre.2002.2421.
88. Daly C, Wong V, Burova E, et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1). Genes Dev 2004;18(9):1060–1071. DOI: 10.1101/gad.1189704.
89. Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci 2002;99(17):11205–11210.
90. Saharinen P, Eklund L, Miettinen J, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell–cell and cell–matrix contacts. Nat Cell Biol 2008;10(5):527–537. DOI: 10.1038/ncb1715.
91. Daly C, Pasnikowski E, Burova E, et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci 2006;103(42):15491–15496. DOI: 10.1073/pnas.0607538103.
92. Valable S, Bellail A, Lesne S, et al. Angiopoietin‐1‐induced phosphatidyl‐inositol 3‐kinase activation prevents neuronal apoptosis. FASEB J 2003;17(3):1–19. DOI: 10.1096/fj.02-0372fje.
93. Chae JK, Kim I, Lim ST, et al. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol 2000;20(12):2573–2578. DOI: 10.1161/01.ATV.20.12.2573.
94. Bierhaus A, Chen J, Liliensiek B, et al. LPS and cytokine-activated endothelium. Semin Thromb Hemost 2000;26(5):571–587. DOI: 10.1055/s-2000-13214.
95. Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukocyte Biol 1999;66(5):698–704. DOI: 10.1002/jlb.66.5.698.
96. Parent C, Eichacker PQ. Neutrophil and endothelial cell interactions in sepsis: the role of adhesion molecules. Infect Dis Clin N Am 1999;13(2):427–447. DOI: 10.1016/s0891-5520(05)70084-2.
97. Petri B, Bixel MG. Molecular events during leukocyte diapedesis. FEBS J 2006;273(19):4399–4407. DOI: 10.1111/j.1742-4658.2006.05439.x.
98. Luscinskas FW, Ma S, Nusrat A, et al. Leukocyte transendothelial migration: a junctional affair. Sem Immunol 2002;14(2):105–113. DOI: 10.1006/smim.2001.0347.
99. Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol 2004;22:129–156. DOI: 10.1146/annurev.immunol.21.090501.080131.
100. Vainer B, Nielsen O. Changed colonic profile of P‐selectin, platelet‐endothelial cell adhesion molecule‐1 (PECAM‐1), intercellular adhesion molecule‐1 (ICAM‐1), ICAM‐2, and ICAM‐3 in inflammatory bowel disease. Clin Exp Immunol 2000;121(2):242–247. DOI: 10.1046/j.1365-2249.2000.01296.x.
101. Lomakina EB, Waugh RE. Adhesion between human neutrophils and immobilized endothelial ligand vascular cell adhesion molecule 1: divalent ion effects. Biophys J 2009;96(1):276–284. DOI: 10.1016/j.bpj.2008.10.001.
102. Kirchhofer D, Tschopp TB, Hadváry P, et al. Endothelial cells stimulated with tumor necrosis factor-alpha express varying amounts of tissue factor resulting in inhomogenous fibrin deposition in a native blood flow system. Effects of thrombin inhibitors. J Clin Invest 1994;93(5):2073–2083. DOI: 10.1172/JCI117202.
103. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics 2016;138(6):e20162013. DOI: 10.1542/peds.2016-2013.
104. Greenberg RG, Kandefer S, Do BT, et al. Late-onset sepsis in extremely premature infants: 2000–2011. Pediatr Infect Dis J 2017;36(8):774. DOI: 10.1097/INF.0000000000001570.
105. Stoll BJ, Hansen NI, Sánchez PJ, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011;127(5):817–826. DOI: 10.1542/peds.2010-2217.
106. Karenberg K, Hudalla H, Frommhold D. Leukocyte recruitment in preterm and term infants. Mol Cell Pediatr 2016;3(1):35. DOI: 10.1186/s40348-016-0063-5.
107. Nussbaum C, Sperandio M. Innate immune cell recruitment in the fetus and neonate. J Reprod Immunol 2011;90(1):74–81. DOI: 10.1016/j.jri.2011.01.022.
108. Nussbaum C, Gloning A, Pruenster M, et al. Neutrophil and endothelial adhesive function during human fetal ontogeny. J Leukocyte Biol 2013;93(2):175–184. DOI: 10.1189/jlb.0912468.
109. Zonneveld R, Jongman R, Juliana A, et al. Low serum angiopoietin-1, high serum angiopoietin-2, and high Ang-2/Ang-1 protein ratio are associated with early onset sepsis in surinamese newborns. Shock (Augusta, Ga.) 2017;48(6):638. DOI: 10.1097/SHK.0000000000000903.
110. Wu KW, Mo JL, Kou ZW, et al. Neurovascular interaction promotes the morphological and functional maturation of cortical neurons. Front Cell Neurosci 2017;11:290. DOI: 10.3389/fncel.2017.00290.
111. Beaino G, Khoshnood B, Kaminski M, et al. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population‐based cohort study. Dev Med Child Neurol 2010;52(6):e119–e125. DOI: 10.1111/j.1469-8749.2010.03612.x.
112. Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92(4):529–534. DOI: 10.1016/s0022-3476(78)80282-0.
113. Heep A, Behrendt D, Nitsch P, et al. Increased serum levels of interleukin 6 are associated with severe intraventricular haemorrhage in extremely premature infants. Arch Dis Childhood-Fetal Neonatal Ed 2003;88(6):F501–F504. DOI: 10.1136/fn.88.6.f501.
114. Leviton A, Allred EN, Dammann O, et al. Systemic inflammation, intraventricular hemorrhage, and white matter injury. J Child Neurol 2013;28(12):1637–1645. DOI: 10.1177/0883073812463068.
115. Borjini N, Sivilia S, Giuliani A, et al. Potential biomarkers for neuroinflammation and neurodegeneration at short and long term after neonatal hypoxic-ischemic insult in rat. J Neuroinflamm 2019;16(1):194. DOI: 10.1186/s12974-019-1595-0.
116. Li S, Liu W, Wang JL, et al. The role of TNF-a, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur Rev Med Pharmacol Sci 2014;18(6):905–909. PMID: 24706318.
117. Yun JH, Han MH, Jeong HS, et al. Angiopoietin 1 attenuates interleukin-6–induced endothelial cell permeability through SHP-1. Biochem Biophys Res Commun 2019;518(2):286–293. DOI: 10.1016/j.bbrc.2019.08.048.
118. Muramatsu K, Fukuda A, Togari H, et al. Vulnerability to cerebral hypoxic-ischemic insult in neonatal but not in adult rats is in parallel with disruption of the blood-brain barrier. Stroke 1997;28(11):2281–2289. DOI: 10.1161/01.str.28.11.2281.
119. Penn JS, Henry MM, Wall PT, et al. The range of PaO2 variation determines the severity of oxygen-induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci 1995;36(10):2063–2070. PMID: 7657545.
120. Connor KM, Krah NM, Dennison RJ, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc 2009;4(11):1565. DOI: 10.1038/nprot.2009.187.
121. Mezu-Ndubuisi OJ. In vivo angiography quantifies oxygen-induced retinopathy vascular recovery. Optom Vis Sci 2016;93(10):1268–1279. DOI: 10.1097/OPX.0000000000000941.
122. Ren Y, Chan HM, Li Z, et al. Upregulation of macrophage migration inhibitory factor contributes to induced N-Myc expression by the activation of ERK signaling pathway and increased expression of interleukin-8 and VEGF in neuroblastoma. Oncogene 2004;23(23):4146–4154. DOI: 10.1038/sj.onc.1207490.
123. Wang J, Lin J, Kaiser U, et al. Absence of macrophage migration inhibitory factor reduces proliferative retinopathy in a mouse model. Acta Diabetol 2017;54(4):383–392. DOI: 10.1007/s00592-016-0956-8.
124. Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol 1996;114(10):1219–1228. DOI: 10.1001/archopht.1996.01100140419009.
125. Shih SC, Ju M, Liu N, et al. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. J Clin Invest 2003;112(1):50–57. DOI: 10.1172/JCI17808.
126. Mezu-Ndubuisi OJ, Wang Y, Schoephoerster J, et al. Intravitreal delivery of VEGF-A165–loaded PLGA microparticles reduces retinal vaso-obliteration in an in vivo mouse model of retinopathy of prematurity. Curr Eye Res 2019;44(3):275–286. DOI: 10.1080/02713683.2018.1542736.
127. Cayabyab R, Ramanathan R. Retinopathy of prematurity: therapeutic strategies based on pathophysiology. Neonatology 2016;109(4):369–376. DOI: 10.1159/000444901.
128. Smith L, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994;35(1):101–111. PMID: 7507904.
129. Stahl A, Connor KM, Sapieha P, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci 2010;51(6):2813–2826. DOI: 10.1167/iovs.10-5176.
130. Mezu-Ndubuisi OJ, Teng P, Wanek J, et al. In vivo retinal vascular oxygen tension imaging and fluorescein angiography in the mouse model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 2013;54(10):6968–6972. DOI: 10.1167/iovs.13-12126.
131. Dailey WA, Drenser KA, Wong SC, et al. Norrin treatment improves ganglion cell survival in an oxygen-induced retinopathy model of retinal ischemia. Exp Eye Res 2017;164:129–138. DOI: 10.1016/j.exer.2017.08.012.
132. Mezu-Ndubuisi OJ, Wanek J, Chau FY, et al. Correspondence of retinal thinning and vasculopathy in mice with oxygen-induced retinopathy. Exp Eye Res 2014;122:119–122. DOI: 10.1016/j.exer.2014.03.010.
133. Mezu-Ndubuisi OJ, Taylor LK, Schoephoerster JA. Simultaneous fluorescein angiography and spectral domain optical coherence tomography correlate retinal thickness changes to vascular abnormalities in an in vivo mouse model of retinopathy of prematurity. J Ophthalmol 2017;2017:9620876. DOI: 10.1155/2017/9620876.
134. Mezu-Ndubuisi OJ, Adams T, Taylor LK, et al. Simultaneous assessment of aberrant retinal vascularization, thickness, and function in an in vivo mouse oxygen-induced retinopathy model. Eye 2019;33(3):363–373. DOI: 10.1038/s41433-018-0205-1.
135. Mitton K, Deshpande M, Wong SC, et al. Retinal plasticity: functional recovery after bipolar cell loss in the oxygen induced retinopathy model. BioRxiv 2019. DOI: 10.1101/2019.12.12.874271.
136. Mezu-Ndubuisi OJ, Macke EL, Kalavacherla R, et al. Long-term evaluation of retinal morphology and function in a mouse model of oxygen-induced retinopathy. Mol Vis 2020;26:257. PMID: 32256029.
137. Mezu-Ndubuisi OJ, Maheshwari A. Role of macrophages in fetal development and perinatal disorders. Pediatr Res 2021;90(3):1–13. DOI: 10.1038/s41390-020-01209-4.
138. Abu El-Asrar AM, Ahmad A, Allegaert E, et al. Interleukin-11 overexpression and M2 macrophage density are associated with angiogenic activity in proliferative diabetic retinopathy. Ocul Immunol Inflamm 2020;28(4):575–588. DOI: 10.1080/09273948.2019.1616772.
139. Mezu-Ndubuisi OJ, Maheshwari A. The role of integrins in inflammation and angiogenesis. Pediatr Res 2021;89(7):1619–1626. DOI: 10.1038/s41390-020-01177-9.
140. Senger DR, Claffey KP, Benes JE, et al. Angiogenesis promoted by vascular endothelial growth factor: regulation through α1β1 and α2β1 integrins. Proc Natl Acad Sci 1997;94(25):13612–13617. DOI: 10.1073/pnas.94.25.13612.
141. Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 1998;125(9):1591–1598. DOI: 10.1242/dev.125.9.1591.
142. Jamali N, Song YS, Sorenson CM, et al. 1,25 (OH) 2D3 regulates the proangiogenic activity of pericyte through VDR‐mediated modulation of VEGF production and signaling of VEGF and PDGF receptors. FASEB Bioadv 2019;1(7):415–434. DOI: 10.1096/fba.2018-00067.
143. Liang W, Jiang L. Relationship between expression of angiopoietin-2 and retinal vascular development in hyperoxic rats. Chin J Pediatr 2009;47(3):204–208.
144. Park YJ, Woo SJ, Kim YM, et al. Immune and inflammatory proteins in cord blood as predictive biomarkers of retinopathy of prematurity in preterm infants. Invest Ophthalmol Vis Sci 2019;60(12):3813–3820. DOI: 10.1167/iovs.19-27258.
145. Woo SJ, Park JY, Hong S, et al. Inflammatory and angiogenic mediators in amniotic fluid are associated with the development of retinopathy of prematurity in preterm infants. Invest Ophthalmol Vis Sci 2020;61(5):42–42. DOI: 10.1167/iovs.61.5.42.
146. Pieh C, Agostini H, Buschbeck C, et al. VEGF-A, VEGFR-1, VEGFR-2 and Tie2 levels in plasma of premature infants: relationship to retinopathy of prematurity. Br J Ophthalmol 2008;92(5):689–693. DOI: 10.1136/bjo.2007.128371.
147. Jobe A, Bancalari E. NICHD/NHLBI. In ORD Workshop Summary. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163(7):1723–1729. DOI: 10.1164/ajrccm.163.7.2011060.
148. Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med 2001;164(10):1755–1756. DOI: 10.1164/ajrccm.164.10.2109111c.
149. Northway Jr WH, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. New Engl J Med 1967;276(7):357–368. DOI: 10.1056/NEJM196702162760701.
150. McEvoy CT, Jain L, Schmidt B, et al. Bronchopulmonary dysplasia: NHLBI workshop on the primary prevention of chronic lung diseases. Ann Am Thorac Soc 2014;11(Suppl 3):S146–S153. DOI: 10.1513/AnnalsATS.201312-424LD.
151. Orfanos S, Mavrommati I, Korovesi I, et al. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. In: Applied physiology in intensive care medicine. Springer; 2006. p. 171–183.
152. Bhatt AJ, Pryhuber GS, Huyck H, et al. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am j Respir Crit Care Med 2001;164(10):1971–1980. DOI: 10.1164/ajrccm.164.10.2101140.
153. Jakkula M, Le Cras TD, Gebb S, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279(3):L600–L607. DOI: 10.1152/ajplung.2000.279.3.L600.
154. Syed M, Das P, Pawar A, et al. Hyperoxia causes miR-34a-mediated injury via angiopoietin-1 in neonatal lungs. Nat Commun 2017;8(1):1–17. DOI: 10.1038/s41467-017-01349-y.
155. Sahni M, Yeboah B, Das P, et al. Novel biomarkers of bronchopulmonary dysplasia and bronchopulmonary dysplasia-associated pulmonary hypertension. J Perinatol 2020;40(11):1634–1643. DOI: 10.1038/s41372-020-00788-8.
156. Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 2004;109(2):159–165. DOI: 10.1161/01.CIR.0000102381.57477.50.
157. Aird WC. Endothelial cell dynamics and complexity theory. Crit Care Med 2002;30(5):S180–S185. DOI: 10.1097/00003246-200205001-00002.
158. Nankervis CA, Reber KM, Nowicki PT. Age-dependent changes in the postnatal intestinal microcirculation. Microcirculation 2001;8(6):377–387. DOI: 10.1038/sj/mn/7800110.
159. Ito Y, Doelle SM, Clark JA, et al. Intestinal microcirculatory dysfunction during the development of experimental necrotizing enterocolitis. Pediatr Res 2007;61(2):180–184. DOI: 10.1203/pdr.0b013e31802d77db.
160. Sabnis A, Carrasco R, Liu SXL, et al. Intestinal vascular endothelial growth factor is decreased in necrotizing enterocolitis. Neonatology 2015;107(3):191–198. DOI: 10.1159/000368879.
161. Yan X, Managlia E, Liu SX, et al. Lack of VEGFR2 signaling causes maldevelopment of the intestinal microvasculature and facilitates necrotizing enterocolitis in neonatal mice. Am J Physiol Gastrointest Liver Physiol 2016;310(9):G716–G725. DOI: 10.1152/ajpgi.00273.2015.
162. Bowker RM, Yan X, Managlia E, et al. Dimethyloxalylglycine preserves the intestinal microvasculature and protects against intestinal injury in a neonatal mouse NEC model: role of VEGF signaling. Pediatr Res 2018;83(2):545–553. DOI: 10.1038/pr.2017.219.
163. Conger JD, Robinette JB, Hammond WS. Differences in vascular reactivity in models of ischemic acute renal failure. Kidney Int 1991;39(6):1087–1097. DOI: 10.1038/ki.1991.138.
164. Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 2002;62(5):1539–1549. DOI: 10.1046/j.1523-1755.2002.00631.x.
165. Kwon O, Phillips CL, Molitoris BA. Ischemia induces alterations in actin filaments in renal vascular smooth muscle cells. Am J Physiol Renal Physiol 2002;282(6):F1012–F1019. DOI: 10.1152/ajprenal.00294.2001.
166. Sutton TA, Kelly KJ, Mang HE, et al. Minocycline reduces renal microvascular leakage in a rat model of ischemic renal injury. Am J Physiol Renal Physiol 2005;288(1):F91–F97. DOI: 10.1152/ajprenal.00051.2004.
167. Schneider R, Raff U, Vornberger N, et al. L-Arginine counteracts nitric oxide deficiency and improves the recovery phase of ischemic acute renal failure in rats. Kidney Int 2003;64(1):216–225. DOI: 10.1046/j.1523-1755.2003.00063.x.
168. Garcia-Criado F, Eleno N, Santos-Benito F, et al. Protective effect of exogenous nitric oxide on the renal function and inflammatory response in a model of ischemia-reperfusion. Transplantation 1998;66(8):982–990. DOI: 10.1097/00007890-199810270-00003.
169. Dagher F, Pollina RM, Rogers DM, et al. The value and limitations of L-arginine infusion on glomerular and tubular function in the ischemic/reperfused kidney. J Vasc Surg 1995;21(3):453–459. DOI: 10.1016/s0741-5214(95)70287-3.
170. Bonauer A, Boon RA, Dimmeler S. Vascular micrornas. Curr Drug Targets 2010;11(8):943–949. DOI: 10.2174/138945010791591313.
171. Crawford Y, Ferrara N. Tumor and stromal pathways mediating refractoriness/resistance to anti-angiogenic therapies. Trends Pharmacol Sci 2009;30(12):624–630. DOI: 10.1016/j.tips.2009.09.004.
172. Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364(7):603–615. DOI: 10.1056/NEJMoa1007374.
173. Wu L, Martínez-Castellanos MA, Quiroz-Mercado H, et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin®): results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol 2008;246(1):81–87. DOI: 10.1007/s00417-007-0660-z.
174. Zhou X, Chen X, Cai JJ, et al. Relaxin inhibits cardiac fibrosis and endothelial–mesenchymal transition via the Notch pathway. Drug Des Dev Ther 2015;9:4599. DOI: 10.2147/DDDT.S85399.
175. Wu M, Tang RN, Liu H, et al. Cinacalcet ameliorates cardiac fibrosis in uremic hearts through suppression of endothelial-to-mesenchymal transition. Int J Cardiol 2014;171(3):e65. DOI: 10.1016/j.ijcard.2013.11.105.
176. Wylie-Sears J, Levine RA, Bischoff J. Losartan inhibits endothelial-to-mesenchymal transformation in mitral valve endothelial cells by blocking transforming growth factor-β-induced phosphorylation of ERK. Biochem Biophys Res Commun 2014;446(4):870–875. DOI: 10.1016/j.bbrc.2014.03.014.
177. Xing K, Murthy S, Liles WC, et al. Clinical utility of biomarkers of endothelial activation in sepsis-a systematic review. Crit Care 2012;16(1):R7. DOI: 10.1186/cc11145.
178. Saldir M, Tunc T, Cekmez F, et al. Endocan and soluble triggering receptor expressed on myeloid cells-1 as novel markers for neonatal sepsis. Pediatr Neonatol 2015;56(6):415–421. DOI: 10.1016/j.pedneo.2015.03.006.
179. Wright JK, Hayford K, Tran V, et al. Biomarkers of endothelial dysfunction predict sepsis mortality in young infants: a matched case-control study. BMC Pediatr 2018;18(1):118. DOI: 10.1186/s12887-018-1087-x.
180. Davis JS, Yeo TW, Piera KA, et al. Angiopoietin-2 is increased in sepsis and inversely associated with nitric oxide-dependent microvascular reactivity. Crit Care 2010;14(3):R89. DOI: 10.1186/cc9020.
181. Maheshwari A, Kelly DR, Nicola T, et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 2011;140(1):242–253. DOI: 10.1053/j.gastro.2010.09.043.
182. Rabinowitz SS, Dzakpasu P, Piecuch S, et al. Platelet-activating factor in infants at risk for necrotizing enterocolitis. J Pediatr 2001;138(1):81–86. DOI: 10.1067/mpd.2001.110132.
183. Schmitt CA, Heiss EH, Dirsch VM. Effect of resveratrol on endothelial cell function: molecular mechanisms. Biofactors 2010;36(5):342–349. DOI: 10.1002/biof.109.
184. Wang K, Lin RZ, Hong X, et al. Robust differentiation of human pluripotent stem cells into endothelial cells via temporal modulation of ETV2 with modified mRNA. Sci Adv 2020;6(30):eaba7606. DOI: 10.1126/sciadv.aba7606.
185. Spiller KL, Anfang RR, Spiller KJ, et al. The role of macrophag phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014;35(15):4477–4488. DOI: 10.1016/j.biomaterials.2014.02.012.
________________________
© The Author(s). 2022 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and non-commercial reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.